INTRODUCTION
Since the early 1970s ozone, as an atmospheric trace gas, has gone from being of interest only to a small group of scientists to an issue of global prominence. This leap occurred because those very same scientists have determined that the normal concentration of atmospheric ozone is under attack from human activities. Its decline was detected from information collected by the WMO Global Ozone Observing System (GO3OS); since the mid- 1950s from more than 150 stations and, in the last 15 years, from a few specialized satellites. Extensive laboratory studies, field measurements and theoretical investigations established a link between humanmade compounds and these ozone losses. It was on the basis of this information that countries, responding to the call by the United Nations Environment Programme (UNEP), signed the first environmental Convention for the Protection of the Ozone Layer (Vienna, 1985).
Ninety-nine per cent of the air we breathe is nitrogen (78%) and oxygen (21%). Their ratio has not changed for millions of years. Rare components such as water vapour, carbon dioxide, methane, nitrous oxide, ozone and inert gases (e.g. argon, helium, neon) make up less than 1% of the volume of air. In every ten million air molecules on average only three are ozone. If all the ozone in the atmosphere was transferred to the Earth's surface, it would assume a thickness of only about 3 mm.
The total ozone in the atmospheric column at any particular place is variable and is mainly determined by large-scale atmospheric dynamics.
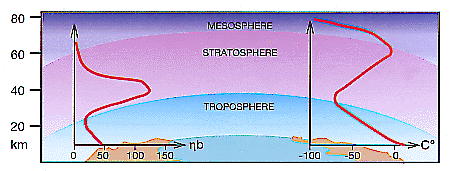
Although exceedingly rare, ozone molecules play a vital role in the life of our planet. They absorb harmful solar ultraviolet radiation (below about 320 nm) shielding us and all other animals and plants from damage. Ozone also largely determines the thermal structure of the stratosphere (10-50 km) where temperature increases with height (Figure 1).
|
|
Figure 1 -- About 90% of the atmospheric ozone is locate in the stratosphere, where it reaches its highest concentration between about 19 and 23 km above the surface of the Earth (left-hand curve). The air temperature, after a rapid decrease with height in the troposphere, increase in the stratosphere because ozone absorbs radiation (right-hand curve) |
Even as the sun's energy produces new ozone, (see box at bottom of page) these gas molecules are continuously destroyed by natural compounds containing oxygen, nitrogen, hydrogen and chlorine or bromine. Such chemicals were all present in the stratosphere long before humans began polluting the air. Nitrogen compounds come from soils and the oceans, hydrogen comes mainly from atmospheric water vapour and chlorine comes from the oceans in the form of methyl chloride and methyl bromide. Now human beings have upset the delicate balance of production and destruction. By releasing additional chlorine- and bromine- containing chemicals (e.g. chlorofluorocarbons) into the atmosphere we have enhanced the destruction of ozone leading to lower ozone concentrations in the stratosphere.
The opposite process is occurring in the lower part of the atmosphere (up to 10- 12 km), called the troposphere. Here, mainly as a result of combustion processes, the local concentrations of ozone in the northern middle latitudes have more than doubled in the last 100 years. This tropospheric ozone increase cannot compensate for the stratospheric decline, but the changes could influence the radiative balance of the earth-atmosphere system.
The subject is being raised on the 50th Anniversary of the United Nations because it represents an environmental success story. Understanding ozone changes and the measures necessary for ozone protection require the collaboration of scientists, governments and industry worldwide. It calls for a common effort by all nations with the collaboration of specialized agencies of the United Nations, such as the United Nations Development Programme and the World Bank, and of other national and international bodies coordinated by WMO and UNEP.
| ATMOSPHERIC OZONE |  |
|
|
Ozone (03) is a form of the element oxygen (0) which has three atoms
in each molecule instead of the two of normal oxygen molecules (02).
It is formed in the stratosphere by the action of solar radiation on
oxygen molecules in a process called photolysis; 02 molecules are broken
down to yield atomic oxygen, which in turn combines with molecular oxygen
to produce ozone. Ozone is destroyed naturally through a series of catalytic cycles involving oxygen, nitrogen, chlorine. bromine and hydrogen. The stratosphere (10-50 km above the earth's surface) contains 90% of all the ozone in the atmosphere. Looking up through the atmosphere, the ozone column has its maximum partial pressure in the lower stratosphere at a level of 19-23 km above the Earth (Figure 1). |
||
 |
The Sun's output and the observed ozone decline | |
|
Stratospheric ozone is primarily created by
ultraviolet (UV) radiation. The Sun's output affects the rate at which
it is produced. The Sun's energy release in the UV part of the spectrum
does vary, especially over the well-known 11-year sunspot cycle. Observations
over several solar cycles since the 1950s show that total global ozone
levels decrease by 1 to 2% from the maximum to the minimum of a typical
solar cycle. The recently -observed long-term changes in ozone are much
greater than this. They cannot be attributed to changes in solar activity.
|
||