More than two years after road access and electrical power to the Mauna Loa Observatory was cut off by lava flows, NOAA staff continue to make critical measurements of the atmosphere and other environmental variables at the remote site.
In 2023, observatory staff installed solar panels at the site and resumed some measurements, including the independent carbon dioxide monitoring programs run by the Global Monitoring Laboratory and Scripps Institution of Oceanography, as well as other atmospheric measurements.
Construction of a temporary road to access the observatory site is anticipated to begin in summer 2025.
Media can contact: Theo Stein (303) 819-7409 (theo.stein@noaa.gov)
Organization(s):



What does this program measure?
We are measuring nitric acid vapor and particles that contain nitrate, sulfate, chloride, ammonium, oxalate, methanesulfonate, calcium, sodium, potassium, and magnesium, which we call “aerosol ions.” We express the results in terms of the mass of each substance per cubic meter of air, µg/m 3. Sometimes we express the concentrations as moles of substance per 1,000,000,000,000 moles of air, “parts per trillion by volume ” (pptv).
How does this program work?
We pull air through a Teflon filter every night. Filters for each day of the week are exposed to an ambient air stream at the 7m level of a 40m MLO tower. We sample from 8 PM to 8 AM every night to provide the maximum probable exposure to the MLO down slope wind regime. Once a week the filters are sent back to our UH laboratory, where the ions are extracted and analyzed using a technique called "ion chromatography." Nitric acid is collected by a nylon filter and analyzed in the same way at UH.
Why is this research important?
These substances have important effects on climate and atmospheric photochemical reactions. We are trying to assess the impact of continental sources on the air of the mid-Pacific, and to define day-to-day and longer term variations (e.g., seasonal) in background atmospheric nitric acid vapor concentrations.
Atmospheric particles affect the amount of sunlight that reaches the Earth, by reflecting (scattering) some of the incoming sunlight back to space. Thus, they often have a cooling effect on climate. Nitric acid and nitrates are a product of both natural sources (lightning) and man-made ones (vehicle exhaust). Sulfates also have natural sources (the ocean and volcanoes) in addition to a large source from coal burning for electric generation. We can sometimes identify the natural and man-made amounts by seeing how much concentrations increase when the air is coming from populated continental regions. However, there are very few measurements of these ions in the Free Troposphere (FT, from about 1 km to 15 km altitude) that can be used to see whether numerical climate models are accurately simulating the impact of OC and EC. Our long-term FT measurements at MLO are being used to test those models and make them more accurate.
Are there any trends in the data?
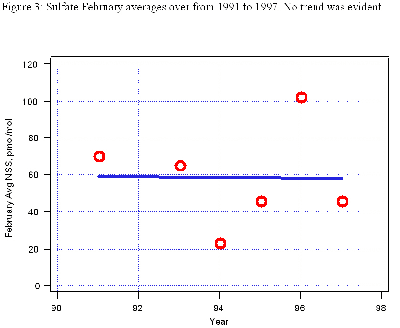
We have seen large year-to-year differences, apparently related to rainout between the source regions and MLO regions in wetter vs. drier years. We have not seen a continual upward or downward trend, though.One of the most interesting trends is the annual variability of these ions.
Figure 1 (below) shows sulfate monthly average observations with three model predictions. The big spring peaks are due to transport from Asia, while the late summer nitrate peak is from North America.
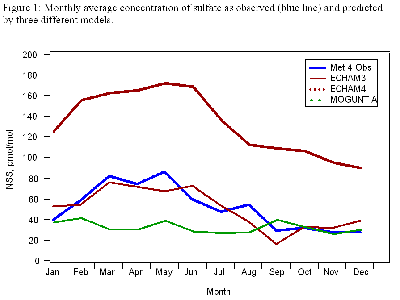
In Figure 2 (below), the daily samples show that Asian dust storms can cause high-concentration events when their calcium-rich particles reach MLO. However, we have not seen long-term trends in any of the ions, in part because the variability from year to year is so great that it masks them.
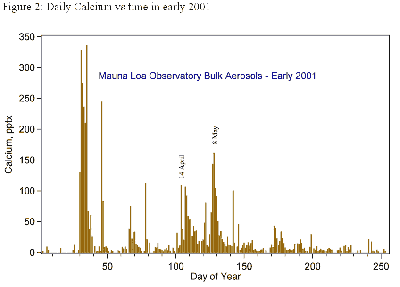
We know from other sources that sulfur emissions from Chinese coal burning are decreasing, even though we could not see a decrease in springtime sulfate at MLO in the 1990s.
See Figure 3 (below).
How does this program fit into the big picture?
What is it's role in global climate change?
Models of climate change must be tested against measurements. One of the largest issues in climate forcing is the role of aerosol particles that scatter sunlight back to space. Our measurements provide one of the few tests of these models’ ability to describe free tropospheric aerosol concentrations. Aerosols in the FT are among the many things that impact the energy balance of the Earth. This project is quantifying one of those impacts so that overall climate models can be made more accurate.
Comments and References
Long-term records of aerosol concentrations are very important assets for testing models. MLO is one of the few places in the world where the mid (free) troposphere can be sampled, so our data set is unique.This work complements our organic aerosol measurements, which have been underway for only a few months. Until recently it was believed that sulfuric acid and sulfate salts were almost the only FT aerosols other than occasional dust from Asia. By measuring both OC and sulfates, we can get a more complete picture of the climatic importance of these particles.
- Kline, J., B. Huebert, S. Howell, B. Blomquist, J. Zhuang, T. Bertram, and J. Carrillo, Aerosol composition and size versus altitude measured from the C-130 during ACE-Asia, J. Geophys. Res., 109 (D19), doi: 10.1029/2004jd004540, 2004.
- Huebert, B.J., C.A. Phillips, L. Zhuang, E. Kjellstrom, H. Rodhe, J. Feichter, and C. Land, Long-term measurements of free-tropospheric sulfate at Mauna Loa: comparison with model simulations, J. Geophys. Res., 106 (D6), 5479-5492, 2001.
- Lee, G., J.T. Merrill, and B.J. Huebert, Variation of free tropospheric total nitrate at Mauna Loa Observatory, Hawaii, J. Geophys. Res., 99 (12821-12831), 1994.
- Lee, G.W., L.Z. Zhuang, B.J. Huebert, and T.P. Meyers, Concentration gradients and dry deposition of nitric acid vapor at the Mauna-Loa Observatory, Hawaii, J. Geophys. Res., 98 (D7), 12661-12671, 1993.
- Norton, R.B., M.A. Carroll, D.D. Montzka, G. Hübler, B.J. Huebert, G. Lee, W.W. Warren, B.A. Ridley, and G. Walega, Measurements of Nitric Acid and Aerosol Nitrate at the Mauna Loa Observatory During MLOPEX 1988, J. Geophys. Res., 97 (D10), 10,415-10,426, 1992.
- Galasyn, J.F., K.L. Tschudy, and B.J. Huebert, Seasonal and diurnal variability of nitric acid vapor and ionic aerosol species in the remote free troposphere at Mauna Loa, Hawaii, J. Geophys. Res., 92 (D3), 3105-3113, 1987.
Lead Investigator(s):
Prof. Barry Huebert (UH)
808-956-6896
John Zhuang
808-956-6091
MLO Contact(s):
Trevor Kaplan
808-933-6965 (x226)
Paul Fukumura
808-933-6965 (x223)
Web Site(s)
http://www.soest.hawaii.edu/oceanography/faculty/huebert.html
Date Started
1985
Related Programs
UHM Corrosion
UHM Organic Carbon
Air Quality Control


