Measuring & Analyzing Greenhouse Gases: Behind the Scenes
Gas Analyzers
The gases are measured using analyzers that use different techniques:
- Infrared absorption - CO2
- Fluoresence - CO
- Gas Chromatography - CH4, N2O, SF6, H2
Infrared Absorption
Remember how carbon dioxide is a greenhouse gas that absorbs infrared radiation and why we want to measure it? Well we can measure CO2 using this very same property of infrared absorption!
 The instrument used is called a Non-Dispersive Infrared analyzer (NDIR).
The air we want to measure is sent into a small chamber, and an infrared light at a specific wavelength is directed into the chamber. We choose a specific wavelength (4.255 microns or 4.255 x 10-6m) that we know is absorbed by CO2, but not by other gases found in air
(Water can also absorb this wavelength of infrared light, so it is important that all the water was removed before the air arrives at the NDIR.)
. At the far end of the chamber is a detector that measures how much infrared light makes it through the air in the chamber. The difference between what we put in and what comes out must have been absorbed by the CO2 in the air inside the chamber. The amount absorbed is proportional to the amount of CO2 in the air, thus less light transmitted means more CO2 in the chamber.
The instrument used is called a Non-Dispersive Infrared analyzer (NDIR).
The air we want to measure is sent into a small chamber, and an infrared light at a specific wavelength is directed into the chamber. We choose a specific wavelength (4.255 microns or 4.255 x 10-6m) that we know is absorbed by CO2, but not by other gases found in air
(Water can also absorb this wavelength of infrared light, so it is important that all the water was removed before the air arrives at the NDIR.)
. At the far end of the chamber is a detector that measures how much infrared light makes it through the air in the chamber. The difference between what we put in and what comes out must have been absorbed by the CO2 in the air inside the chamber. The amount absorbed is proportional to the amount of CO2 in the air, thus less light transmitted means more CO2 in the chamber.
Fluoresence
 Carbon monoxide is measured using CO’s fluorescent ability. In the CO analyzer, we shine ultra-violet light at a specific wavelength onto a chamber filled with our sample air. The CO absorbs that ultra-violet light and then re-emits it as fluorescence, at a longer wavelength specific to CO. The amount of fluorescence is measured using a special detector and is proportional to the amount of CO in the sample air. CO is not the only thing that has fluorescent properties – glow-in-the-dark toys work the same way, absorbing normal visible light, then slowly re-emitting that light at a specific wavelength (that greenish color you see is one specific wavelength).
Carbon monoxide is measured using CO’s fluorescent ability. In the CO analyzer, we shine ultra-violet light at a specific wavelength onto a chamber filled with our sample air. The CO absorbs that ultra-violet light and then re-emits it as fluorescence, at a longer wavelength specific to CO. The amount of fluorescence is measured using a special detector and is proportional to the amount of CO in the sample air. CO is not the only thing that has fluorescent properties – glow-in-the-dark toys work the same way, absorbing normal visible light, then slowly re-emitting that light at a specific wavelength (that greenish color you see is one specific wavelength).
It works in a similar way with CO, except that the wavelengths that CO absorbs and re-emits at are different than for glow-in-the-dark toys.
Gas Chromatography
The remaining four gases all have to be separated from the rest of the air before we can measure them, and for this we use a gas chromatograph. Gas chromatography is the process of separating gases by using the fact that they flow at different rates. A short experiment helps us understand this concept.
Short Experiment:
- A paper towel
- Several different types of markers: Not the waterproof kind. (to check this, draw a small dot on a piece of scratch paper and see if water will smear it.)
- A flat tray with less than 0.5 inch of water in it
- Draw dots smaller than the size of a pea approximately 1 inch from the bottom of the paper and 1 inch apart. It is very important that the dots are not at the very bottom of the paper, and we will see why very shortly!
- Dip the bottom edge of the paper in to the tray of water, making sure that the dots do not touch the water.
- Hold the paper in the water for a few minutes. Watch what happens as the water moves up the paper. What do you see? What is the ink doing?


The ink should move up the paper with the water, but should separate as it moves. The separation of the ink depends on how strongly the different color molecules stick to the paper.
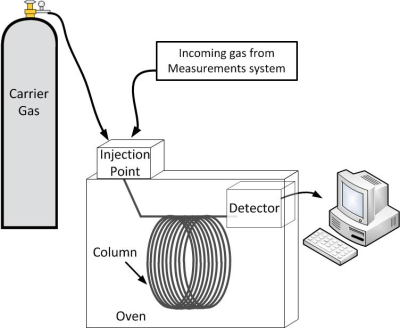
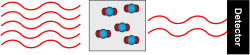
Gas chromatography uses a similar principle to separate gases in an air sample. Instead of using water as a “carrier,” gas chromatography uses an inert gas carrier, and the paper towel is replaced by a very long, very thin hollow tube with a sticky coating. Our sample air flows inside the tube, coated with the sticky substance.
Different gases flow faster than others, depending on how much they stick to the coating. The type of carrier gas, length of the tube, and the kind of sticky substance used can be adjusted depending on what compounds are being measured. The system is tested in the laboratory with artificial air mixtures to figure out how long it takes for each gas to flow through it. Once we know when to expect the gas we want to measure to emerge from the tube, it is directed to a detector designed specifically for that gas at the correct time. The detector is designed to ionize the compound of interest, and the electrons either create a current, or disturb a constant current in order to create a signal that can be related to the amount of compound present.
We use two different gas chromatographs, and 3 different types of detectors to allow complete separation of the gases we are interested in.
- CH4 is detected using a Flame Ionization Detector - This consists of a hydrogen-oxygen flame which heats the sample until it is ionized.
- H2 is detected using a Helium Pulse Discharge Detector - A very large voltage is directed at the sample for a very short period of time. Some of the hydrogen molecules are ionized by the voltage, some by the ionized helium molecules.
- SF6 and N2O are detected using an Electron Capture Detector - The Electron Capture Detector (ECD) uses nickel-63, a radioactive beta (electron) emitter that ionizes the carrier gas resulting in an electric current. A gas like SF6 captures some of the electrons thereby reducing the current when it enters the detector.
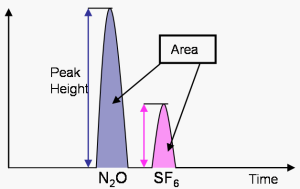
The resulting current that is recorded in peaks (after changing dips into peaks) on what is called a Chromatogram. The different peaks correspond to the concentrations of the different gases in the sample, and are analyzed by taking the area under the curve. The area under the curve is compared with the area under curve for the standards with known mole fraction values measured in the same chromatograph. In this way the area under the curve is calibrated so that it corresponds to mole fractions for the compounds measured.
Next: Measurement Standards
 Previous
Previous