More than two years after road access and electrical power to the Mauna Loa Observatory was cut off by lava flows, NOAA staff continue to make critical measurements of the atmosphere and other environmental variables at the remote site.
In 2023, observatory staff installed solar panels at the site and resumed some measurements, including the independent carbon dioxide monitoring programs run by the Global Monitoring Laboratory and Scripps Institution of Oceanography, as well as other atmospheric measurements.
Construction of a temporary road to access the observatory site is anticipated to begin in summer 2025.
Media can contact: Theo Stein (303) 819-7409 (theo.stein@noaa.gov)
NOAA Cooperative Global Air Sampling Network - Greenhouse Gases
Organization(s):
 National Oceanic and Atmospheric Administration (NOAA),
National Oceanic and Atmospheric Administration (NOAA),
Global Monitoring Laboratory (GML)
What does this program measure?
The NOAA GML CCGG cooperative global air sampling network is an international effort, and includes regular discrete air flask samples from both Mauna Loa Observatory and Cape Kumukahi, Hawaii. Complete information about the program is available at https://gml.noaa.gov/ccgg/flask.html.
The program measures the following (where ppm is parts-per-million, ppb is parts-per-billion, ppt is parts-per-trillion, pm is per-million, and per mil is per-thousand):
| Measurement | Chemical Formula | Units |
|---|---|---|
| Carbon Dioxide | CO2 | ppm |
| Methane | CH4 | ppb |
| Carbon Monoxide | CO | ppb |
| Hydrogen | H 2 | ppb |
| Nitrous Oxide | N2O | ppb |
| Sulfur Hexaflouride | SF6 | ppt |
| isotopic ratio of carbon dioxide | Carbon-13 / Carbon-12 | per mil |
| isotopic ratio of carbon dioxide | Oxygen-18 / Oxygen-16 | per mil |
How does this program work?
Flask samples are taken at Mauna Loa Observatory and Cape Kumukahi, Hawaii, and are sent to the NOAA GML laboratory in Boulder, Colorado. They are then analyzed with the following techniques:
- Carbon dioxide (CO2) is measured by cavity ring-down spectroscopy (CRDS);
- Methane (CH4) is measured by gas chromatography (GC) with flame ionization detection;
- Carbon monoxide (CO) is measured by GC with mercuric oxide (HgO) reduction detection;
- Hydrogen (H2) is also measured by GC with HgO detection;
- Nitrous Oxide (N2O) and sulphur hexafluoride (SF6) use GC with electron capture detection;
- Carbon-13 (13C) / Carbon-12 (12C) and isotopes 18O / 16O use mass spectrometry.
Why is this research important?
In order to determine temporal and spatial variations in the global atmosphere, deduce better estimates of sources and sinks, and better understand the carbon cycle.
Are there any trends in the data?
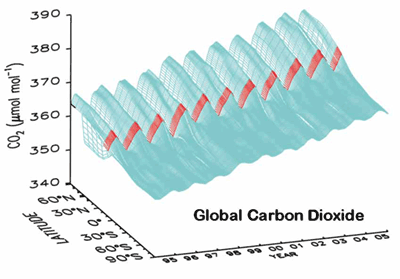
Carbon dioxide is increasing by ~1.5 parts per million per year;
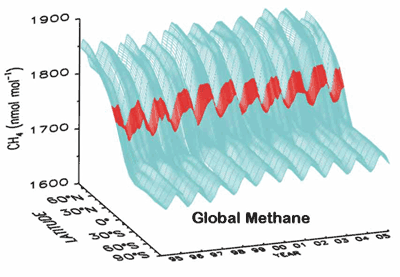
Methane was increasing, but has been nearly constant for the past 2 years;
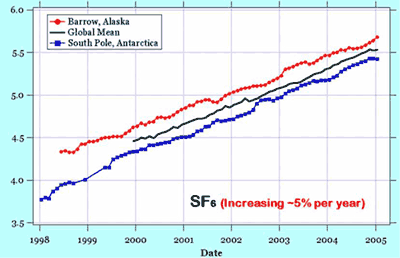
Nitrous oxide and sulphur hexaflouride are increasing. Sulfur hexaflouride (SF6) is the most potent greenhouse gas on a molecule-to-molecule basis, and has an atmospheric lifetime of about 3,000 years! But, since SF6 concentrations are relatively low at present, their greenhouse effet will not be felt for many years. SF6 is used in the electrical power transmission industry and its sources are mainly in the northern hemisphere.
Carbon-13 / Carbon-12 is decreasing.
What is it's role in global climate change?
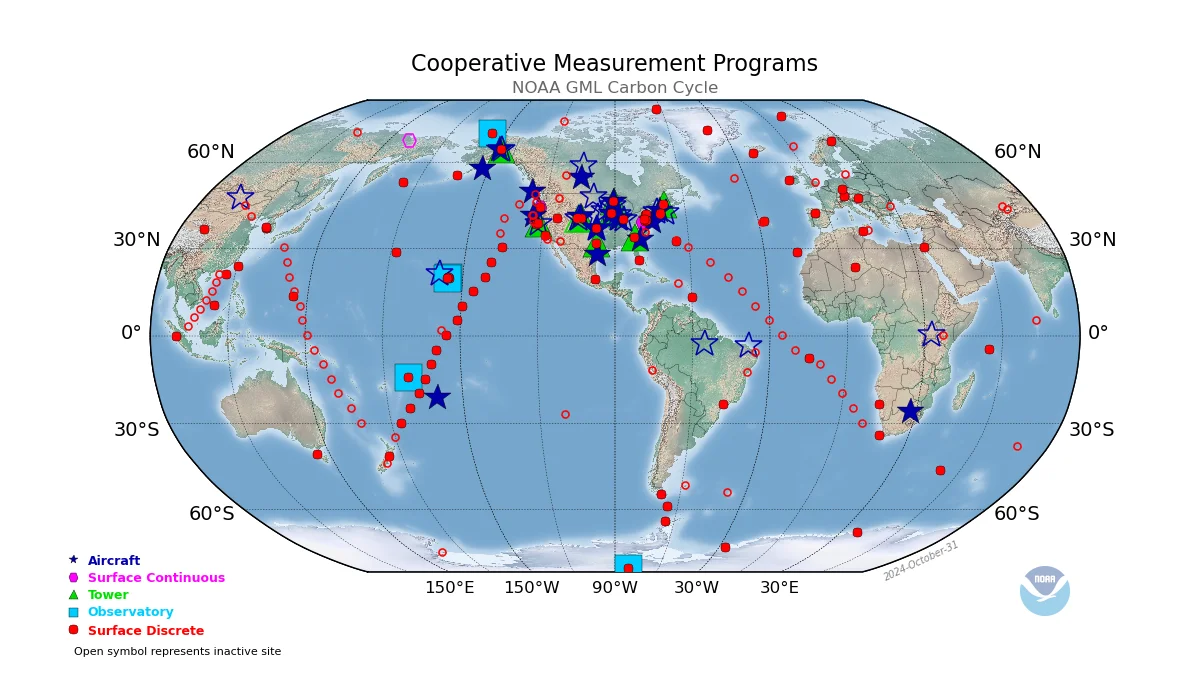
The CCGG flask sample network consists of the sites shown on the map below (click map to enlarge).
Carbon dioxide is the most important anthropogenic “greenhouse gas” (it produces about 60% of present day greenhouse gas climate forcing), followed by methane (about 20%). Nitrous oxide and sulphur hexaflouride are also infrared absorbers. Carbon monoxide affects the greenhouse gases through its atmospheric chemistry. A better understanding of the carbon cycle is crucial to make informed decisions about future climate and energy policies.
Comments and References
More information, plus many figures, are available from the CCGG web site.
Lead Investigator(s):
Arlyn Andrews
720-213-6177
John Miller
720-213-8626
MLO Contacts(s):
Aidan Colton
808-933-6965 (x233)
Matthew.Martinsen
808-933-6965 (x228)
Web Site(s):
https:/gml.noaa.gov/
/ccgg/index.html
Date Started:
08/20/1969
Related Programs
In Situ Carbon Monoxide
In Situ Carbon Dioxide
In Situ Methane
Greenhouse Gases