More than two years after road access and electrical power to the Mauna Loa Observatory was cut off by lava flows, NOAA staff continue to make critical measurements of the atmosphere and other environmental variables at the remote site.
In 2023, observatory staff installed solar panels at the site and resumed some measurements, including the independent carbon dioxide monitoring programs run by the Global Monitoring Laboratory and Scripps Institution of Oceanography, as well as other atmospheric measurements.
Construction of a temporary road to access the observatory site is anticipated to begin in summer 2025.
Media can contact: Theo Stein (303) 819-7409 (theo.stein@noaa.gov)
NOAA Halocarbon Flask Sampling at Mauna Loa & Cape Kumukahi, Hawaii
Organization(s):
 National Oceanic
and Atmospheric Administration (NOAA),
National Oceanic
and Atmospheric Administration (NOAA),
Global Monitoring Laboratory (GML)
What does this program measure?
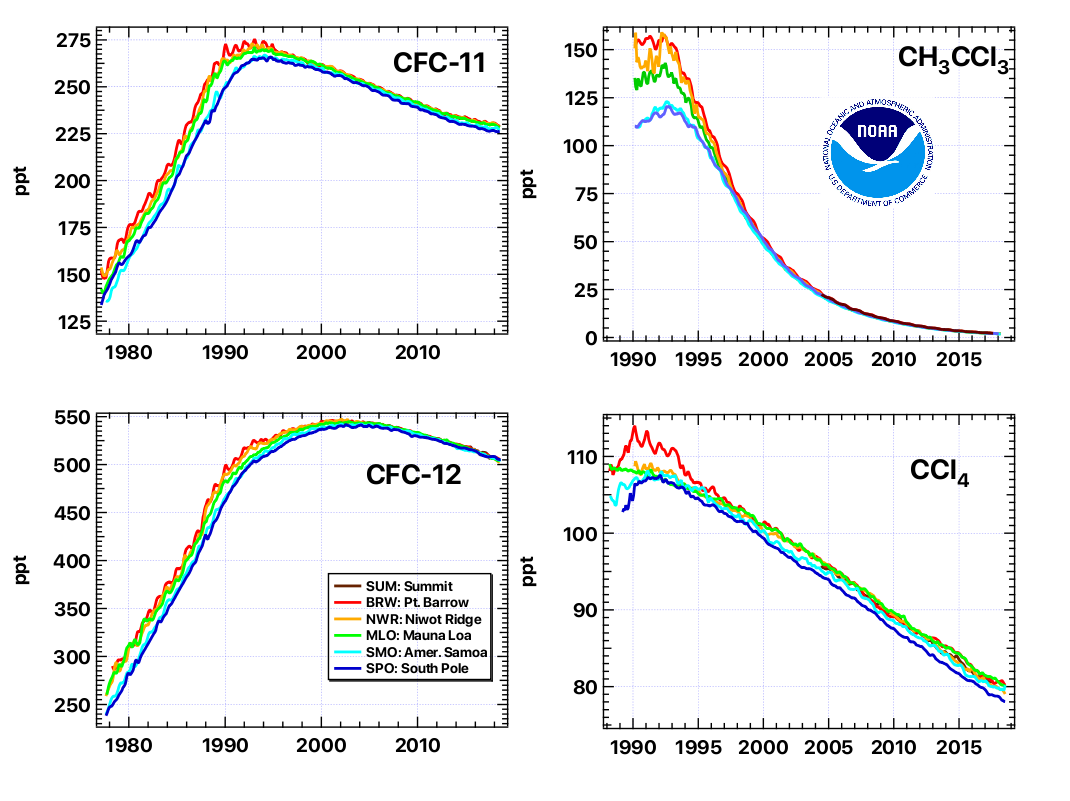
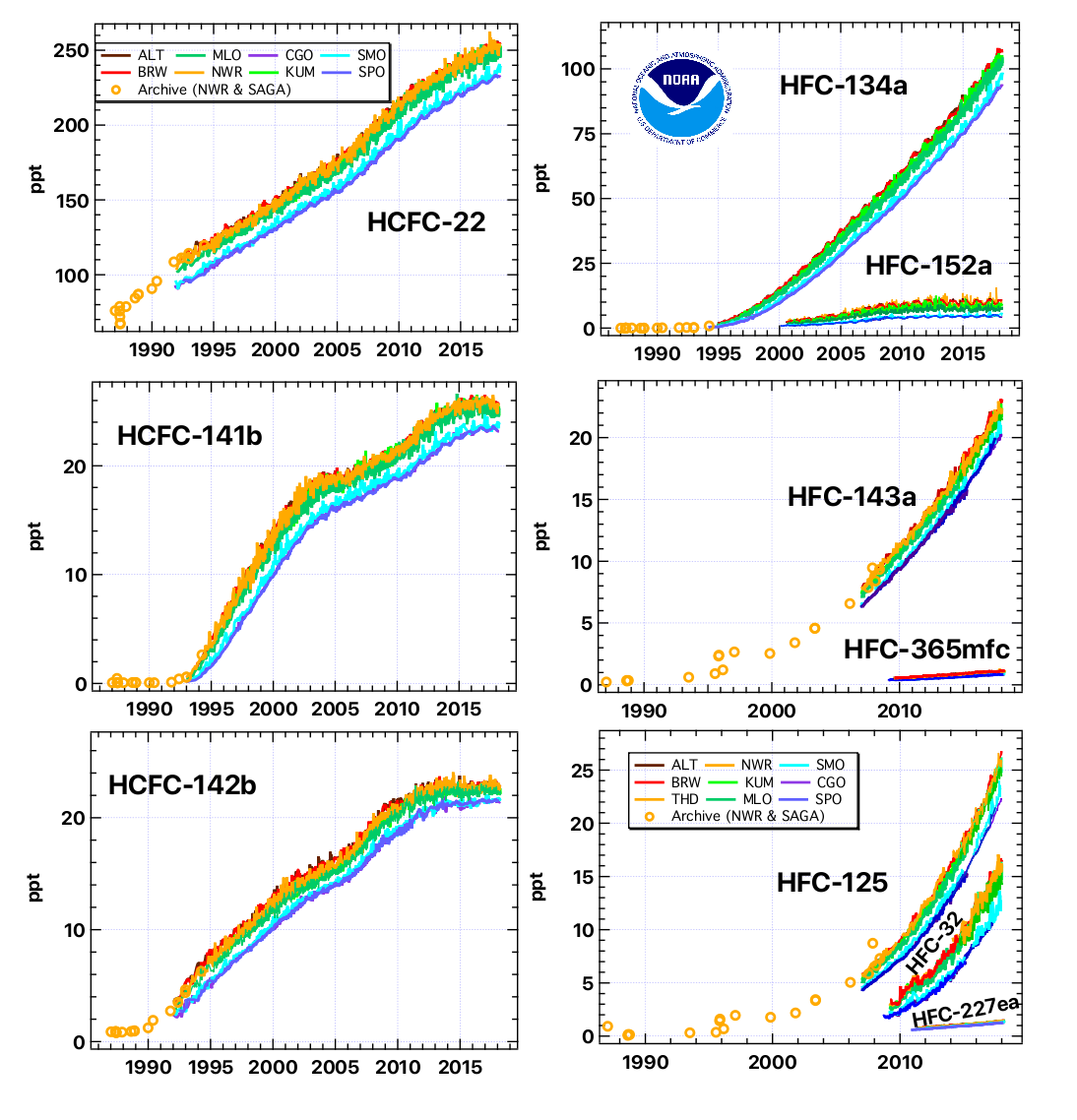
The Mauna Loa Halocarbon and Trace Species (HATS) program is a cornerstone to NOAA's efforts to monitor ozone-depleting gases across the globe at remote sites. The NOAA record of ozone-depleting gases is one of the world's most reliable and complete records of atmospheric changes in ozone-depleting gases, and their replacements. Many gases measured in the HATS group also influence climate, such as N2O and SF6, among others. And many are considered hazardous air pollutants.
The table below contains a list of chemicals for which data is currently being obtained from the HATS flask sampling program. The column labeled "DL" denotes the detection limit measured in parts-per-trillion (ppt). The column labeled "Relevance" is clarified by the footnotes below the table.
| CFCs Chlorofluorocarbons | Relevance* | DL |
|---|---|---|
| CFC-11 - Trichloro(mono)fluoromethane | 1,2 | 0.5 |
| CFC-12 - Dichlorodifluoromethane | 1,2 | 0.5 |
| CFC-113 - Trichlorotrifluoroethane | 1,2 | 0.5 |
| CFC-114 / CFC-114a § | 1 | <0.5 |
| HCFCs Hydrochlorofluorocarbons | Relevance* | DL |
| HCFC-22 - Chlorodifluoromethane | 1,2,3 | <0.5 |
| HCFC-141a - Dichlorofluoroethane | 1,2 | 0.15 |
| HCFC-142b - Chlorodiflouroethane | 1,2 | <0.5 |
| HCFC-124 § | 1,3 | <0.5 |
| HFCs | Relevance* | DL |
| HFC-134a | 2,3 | <0.25 |
| HCFC-152a | 3 | 0.5 |
| Methyl Halides | Relevance* | DL |
| CH3Cl | 1,4 | 0.5 |
| CH3Br - Methyl Bromide | 1,4 | 0.1 |
| CH3I | 4 | 0.1 |
| Other Chlorinated Hydrocarbons | Relevance* | DL |
| CCl 4 - Carbon Tetrachloride | 1,4 | 0.5 |
| CH 3CCl 3 - Methyl Chloroform | 1,4 | 0.25 |
| CH2Cl2 - Dichloromethane | 4 | 0.5 |
| CHCl3 - Chloroform | 4 | 0.5 |
| C2Cl4 - Tetrachloroethylene | 4 | <0.25 |
| C2HCl3 - Trichloroethylene | 4,5 | <0.5 |
| Other Brominated Hydrocarbons | Relevance* | DL |
| Halon 1211 | 1 | 0.1 |
| Halon 1301 | 1,5 | 0.1 |
| Halon 2402 | 1,5 | 0.1 |
| CHBr3 - Bromoform | 4 | 0.1 |
| CH2Br2 | 4,5 | <0.2 |
| Miscellaneous | Relevance* | DL |
| C6H6 - Benzene | 4 | <0.5 |
| COS - Carbonyl Sulfide | 4 | 1.0 |
| CS2 | 4 | <0.5 |
| N2O - Nitrous Oxide | 1,2 | n.d. |
| SF6 | 2 | n.d. |
§ = Not well calibrated
*1 = Primary ozone-depleting gas, production regulated by the Montreal Protocol (with the exception of
CH3Cl and N2O)
*2= Radiatively important gas
*3 = Replacement for ozone-depleting gases
*4 = Hazardous Air Pollutant
*5 = Measured from a subset of samples collected
How does this program work?
Flasks are sampled with air from atop MLO and at other sites across the globe. These flasks are then sent to Boulder and are analyzed by gas chromatography with electron capture and mass spectrometric detection techniques
The table below lists the instruments, frequency and location of the specific compounds measured.
| Measurements | Instruments | Frequency | Site |
|---|---|---|---|
| N2O, CFC-11, CFC-12, CFC-113, CH3CCl3, CCl4 | 300-mL stainless steel flasks | 1/week | MLO |
| N2O, CFC-11, CFC-12, CFC-113, CH3CCl3, CCl4, SF6, HCFC-22, HCFC-141b, HCFC-142b, CH3Br, CH3Cl, CH2Cl2, CHCl3, C2HCl3, C2Cl4, H-1301, H-1211, H-2402, HFC-134a | 850-mL stainless steel flasks | 1/week | MLO & Kumukahi |
| N2O, CFC-11, CFC-12, CFC-113, CH3CCl3, CCl4 | HP5890 automated GC | 1/hour | MLO |
| N2O | Shimadzu automated GC | 1/hour | MLO |
Why is this research important?
This program provides the basis for understanding changes in the depletion of the stratospheric ozone layer. The data is heavily relied upon by nations across the globe to understand if policy decisions have had the desired effect in reducing atmospheric amounts of chemicals that deplete the ozone layer. Nearly all the chemicals measured are also strong greenhouse gases and influence climate. The results are also important for understanding the concentrations of hazardous air pollutants being transported from other countries to the US.
Are there any trends in the data?
The results from this program were the first to show that the overall abundance of ozone-depleting gases peaked in the early-mid 1990s and has since declined. This bodes well for the ozone layer--if these declines continue, scientists expect that the ozone layer will ultimately recover. Despite a decline in the ozone-depleting power of these gases, their influence on warming the climate continues to increase.
Note: For more information and results, please read Halocarbons and Other Atmpsoheric Trace Species (HATS) Trends in the Troposphere (pdf)
What is it's role in global climate change?
This measurement program supplies unique and important information that enables humans everywhere to understand how the threat that human actions have posed to the ozone layer and climate are changing over time.
Comments and References
- Butler, J.H., K.B. Egan, T.M. Thompson, and J.W. Elkins, Nitrous oxide in the atmosphere, surface water, and at depth for the western Pacific and Eastern Indian Oceans, in EOS, pp. 1220, 1987.
- Butler , J.H., J.W. Elkins, B.D. Hall, S.O. Cummings, and S.A. Montzka, A decrease in the growth rates of atmospheric halon concentrations, Nature, 359, 403-405, 1992.
- Butler, J.H., J.W. Elkins, T.M. Thompson, and K.B. Egan, Tropospheric and dissolved N2O of the west Pacific and east Indian Oceans during the El Niño-Southern Oscillation event of 1987, J. Geophys. Res. , 94 (D12), 14865-14877, 1989.
- Butler, J.H., S.A. Montzka, A.D. Clarke, J.M. Lobert, and J.W. Elkins, Growth and distribution of halons in the atmosphere, J. Geophys. Res. , 103 (D1), 1503-1511, 1998.
- Elkins, J.W., T.M. Thompson, T.H. Swanson, J.H. Butler, B.D. Hall, S.O. Cummings, D.A. Fisher, and A.G. Raffo, Decrease in the growth rates of atmospheric chlorofluorocarbons
- Montzka, S.A, J.H. Butler, B.D. Hall, J.W. Elkins, D.J. Mondeel, A decline in tropospheric organic bromine, Geophy. Res. Lett., 30(15), 1826, doi:10.1029/2003GL017745, 2003.
- Montzka, S.A., J.H. Butler, J.W. Elkins, T.M. Thompson, A.D. Clarke, and L.T. Lock, Present and future trends in the atmospheric burden of ozone-depleting halogens, Nature, 398, 690-694, 1999.
- Montzka, S.A., J.H. Butler, R.C. Myers, T.M. Thompson, T.H. Swanson, A.D. Clarke, L.T. Lock, J.W. Elkins, Decline in the tropospheric abundance of halogen from halocarbons: Implications for stratospheric ozone depletion, Science, 272, 1318-1322, 1996 (received NOAA Outstanding Scientific Paper of the Year Award).
- Montzka, S.A., R.C. Myers, J.H. Butler, J.W. Elkins, L. Lock, A. Clarke, and A.H. Goldstein, Observations of HFC-134a in the remote troposphere, Geophys Res. Lett., 23, 169-172, 1996.
- Montzka, S.A., R.C. Myers, J.H. Butler, and J.W. Elkins, Early trends in the global tropospheric abundance of hydrochlorofluorocarbon-141b and -142b, Geophys. Res. Lett., 21, 2483-2486, 1994.
- Montzka, S.A., R.C. Myers, J.H. Butler, J.W. Elkins, and S.O. Cummings, Global tropospheric distribution and calibration scale of HCFC-22, Geophys Res. Lett., 20, 703-706, 1993.
Lead Investigator(s):
MLO Contact(s):
Aidan Colton
808-933-6965 (x233)
Paul Fukumura
808-933-6965 (x223))
Web Site(s)
https://gml.noaa.gov/hats
Halocarbons and Other Atmpsoheric Trace Species (HATS)
Trends in the Troposphere (pdf)
Date Started
1977
Related Programs
Stealth Gas Chromatograph
Cooperative Global Air Sampling
Greenhouse Gases