The Measurements: Accelerator Mass Spectrometry for 14C
After the Stable Isotope Lab
After a complete analysis for greenhouse gases and stable isotopes, the air flasks make their final stop at the Accelerator Mass Spectrometry (AMS) Radiocarbon Laboratory. Located just down the hall from the stable isotope laboratory, this lab is also part of INSTAAR. Here, the last of the air remaining in the sample flask is used to determine the Δ14C value.

Because 14C is so rare (for every 1,000,000,000,000 carbon atoms, only one of them is 14C), more air is needed to accurately find the Δ14C of the sample (about 2 liters of air, to be precise – the size of a family-sized soda bottle). For this reason, the remainder of the air from both sample flasks is combined into one sample (Still, to ensure the data quality, NOAA scientists collect an extra pair of flasks at some sites so they can still compare measurements from two different samples collected at the same time and place). The air sample is extracted from the flask and then, just as in the stable isotope laboratory, the carbon dioxide must be separated from the water vapor and other gases in the air sample. This is done using a purification system much like that at the stable isotope laboratory, but because more air is needed, it is a larger system.
Purification
Sample purification occurs on a machine nicknamed “CRex.” Although it does lack the teeth that would make a T-Rex terrifying, at first glance CRex gives you plenty of reasons to step away. A liquid nitrogen tank, kept at a freezing -196°C (-321 °F), sits to the side and feeds liquid nitrogen into the purification system. At times (such as in the picture above) the liquid nitrogen in the system is replaced and splashes over the container. When it's time to remove the completed samples from CRex, the lab technician seals them inside glass tubes using a gas torch. Also, during one step, there is a possibility of producing liquid oxygen–which is so explosive it is used for space rockets!
Just like in the initial step for isotope ratio mass spectrometry, the lines in the machine (through which the sample will later pass) need to first be evacuated of air. This is also done using a vacuum line that removes all leftover air so that the sample does not become contaminated when passing through the machine. This step is comparable to “cleaning” the machine. You don't want any left over air getting in your sample, just like you wouldn't want any food leftovers in your gourmet dinner that you are cooking for houseguests.

Once the line is evacuated, a flask valve is opened to introduce the air from the sample into the line, without contamination from the air in the room. This air is gradually pulled through the line using the vacuum pump and a special valve to control the flow rate–or how fast the air travels through the line.
After extracting the sample, the first step is to remove water from the air sample using a water “trap”. This is simply three loops in the vacuum line that are immersed in a bucket filled with very cold liquid kept at -90°C (-130 °F). As the air passes through this trap, the water freezes onto the walls of the line as ice, and is left behind. Although water freezes at 0°C (32°F), the water trap must be much colder to ensure that every last bit is removed.
Water, of course, does not contain carbon, but nevertheless it is important to remove it because it can affect the precision of the isotopic reading or can even interfere with the graphite reaction, which comes in a few steps...
Next, the carbon dioxide is captured and isolated from the rest of the air, using the CO2 trap. Like the water trap, it is three loops in the line, but this time they are immersed in liquid nitrogen, at -196°C (-321 °F). At this temperature, CO2 freezes out as solid CO2 (dry ice) onto the walls of the line. The three loops ensure that all of the CO2 is collected, and none stays as a gas. The rest of the air (nitrogen, oxygen, etc.) will not freeze at this temperature, and passes onwards to the vacuum pump and out into the room. The one exception is nitrous oxide, which has a very similar freezing point to CO2, and is collected along with the CO2. Since it does not contain any carbon, and there is much less of it than CO2, NOAA scientists do not worry about it. It is, however, very important to make sure that some of the other gases are not frozen. If we flow the air through the traps at too fast a rate, oxygen and methane could also be captured in the liquid nitrogen. Methane contains carbon, so it could contaminate our sample. Oxygen doesn't contain carbon, but liquid oxygen is highly explosive!!!
Once all of the air has passed through the system, the frozen CO2 is left behind. The valves are closed off so that the CO2 cannot be sucked away to the vacuum pump, and the liquid nitrogen is also removed from around the CO2 trap. The trap gradually warms up to room temperature, and the solid CO2 evaporates back into CO2 gas. Instead of waiting for the sample to naturally heat up and return to a gaseous phase, the laboratory uses a simple fan–just like you could buy at the store–to quicken the process so that more samples can be extracted in less time. The amount of CO2 gas collected is measured and checked to make sure it matches the CO2 concentration that was measured earlier (in another lab…before the samples came to INSTAAR) from some of the air in the same flask (see the page Collection and Analysis). The computer system controlling CRex calculates the amount of sample using basic physical relationships between pressure, volume, temperature, and the amount of sample. Finally, the CO2 gas is transferred into a small glass tube, and sealed up for the next step. This is done by evacuating the glass tube and vacuum line using the vacuum pump. Then liquid nitrogen is placed around the glass tube, and the CO2 sample is allowed to enter the glass tube. After a few minutes, all the CO2 freezes into the tube. A welder's torch is used to melt the glass and seal off the tube, with the frozen CO2 inside. Once the liquid nitrogen is removed, the CO2 returns to gas phase, and the tube of CO2 can be stored indefinitely–or reduced to form graphite.

A Change to Graphite
To measure the 14C isotopic composition, the carbon from the carbon dioxide sample must be converted to graphite (pure carbon). This is done by mixing the carbon dioxide with hydrogen gas and cooking the mixture at yet another extreme temperature. This time, the sample is heated at 625°C (1,157 °F) for several hours.

An iron catalyst is also needed to ensure that the proper reaction occurs – a small amount of iron powder is placed inside the reaction vessel at the start of the process. The products of the reaction are graphite powder, which forms on the iron powder as a black solid, and water, which is frozen into ice using a water trap. This water trap uses the same concept as the one used for the extraction procedure, but does use a different technology.

The reaction is monitored by checking the pressure: both the carbon dioxide and hydrogen used in the reaction are gases, but the products, graphite and water, both end up as solids. So, as the reaction progresses, the pressure inside the reaction vessel goes down. When the reaction is complete, only a small amount of gas remains – leftover hydrogen that was not used in the reaction.
Once the reaction is completed, the graphite/iron mixture is packed into a small aluminium “target” which is analyzed using accelerator mass spectrometry. From the two liters of air that we started with, only about 0.4 milligrams of graphite is produced, but this is enough to make a very precise measurement.

The Final Step
Analyzing a single air sample for its isotopic content is truly a multistep process that takes place across the United States–if not the globe. Not only do the samples come from around the world, but they also travel yet again in the final step. At this stage, the graphite targets must be measured using an accelerator mass spectrometer (AMS). NOAA samples are measured using one of several different AMS labs, usually the Keck Carbon Cycle AMS at the University of California, Irvine or the Center for Accelerator Mass Spectrometry at Lawrence Livermore National Labs in Livermore, California. Some samples are even sent to a lab as far away as the Rafter Radiocarbon Laboratory in New Zealand.
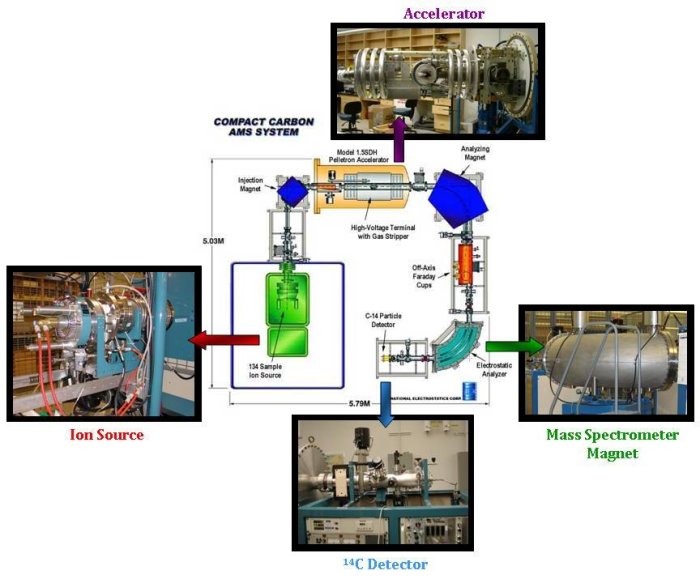
The accelerator mass spectrometer consists of three main parts:- An ion source, where the graphite targets are bombarded with cesium atoms to produce negatively charged ions of carbon.
- A particle accelerator. The negatively charged carbon ions are injected into the particle accelerator. This step is used to remove nitrogen atoms and other particles that have the same mass as 14C (14N has almost identical mass as 14C, and as 80% of the atmosphere, it would be impossible to measure 14C without first removing all 14N). These negative ions are then accelerated through foil or gas strippers, which produce positive ions that are separated and then detected.
- A mass spectrometer. Once the ions have passed through the particle accelerator, they arrive at a large magnet, which deflects the ions on a different trajectory, depending on their mass. Detectors are set in the right places to capture and measure the 12C, 13C and 14C ions. The 12C and 13C ions are measured in the same way as the stable isotope mass spectrometer, using Faraday cups. 14C however, requires a special detector that counts the individual 14C ions as they arrive.
The ratio of 14C to 12C is calculated from the measurement, and compared to standard materials with known 14C content to obtain Δ14C. Finally, a vital part of the process is to remember the three C's: careful, consistent, and continual. Tanks of air with a known amount of 14C are regularly measured along with real samples to ensure the quality of the data. Throughout the whole process, the machines are monitored to ensure that they are functioning properly.
To read about the importance of studying 14C from the Lawrence Livermore National Laboratory's Center for Accelerator Mass Spectrometry, visit:
https://cams.llnl.gov/naturalcarbon.php?id=8
Or visit the University of California, Irvine Laboratory's website at:
http://www.ess.uci.edu/ams/
The Steps Condensed
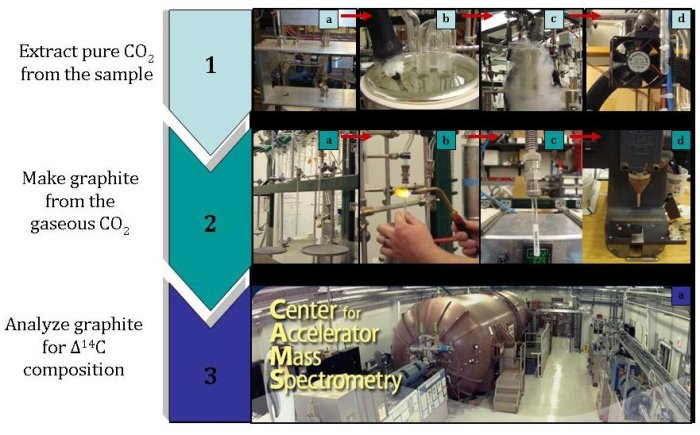
1a) Release air from the paired air flasks into the vacuum line.
1b) Remove water vapor using a water trap.
1c) Separate carbon dioxide from other gases by freezing the carbon dioxide using liquid
nitrogen.
1d) Warm the pure carbon dioxide sample so that it is again in gaseous form.
2a) Move the sample from the purification system to a storage glass vial.
2b) Seal the glass vial that contains the carbon dioxide sample.
2c) Reduce the carbon dioxide to make graphite by using hydrogen and an iron catalyst.
2d) Pack the graphite sample into targets and send the targets to an accelerator mass
spectrometry lab.
3a) Use an accelerator mass spectrometer to accelerate negative ions, which are created
from the graphite sample. These ions are then separated based on their masses. Since
there are so few 14C atoms, these atoms are counted individually and compared to a
standard to find the Δ14C of the sample.
3b) Check the data quality.

 Previous
Previous
