The Technical Details: Chemistry
Composition of an Atom
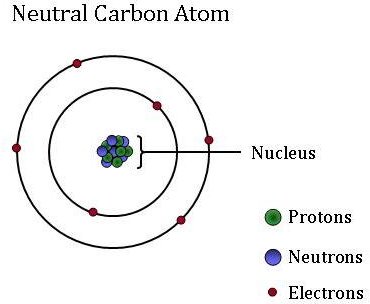
Atoms, which are the basic, fundamental unit of all matter, can differ greatly from one another. Although atoms are too small to see without using high-powered microscopes, they are composed of even smaller particles: protons, neutrons, and electrons.
Electrons, which are extremely light, negatively-charged particles, orbit around a central mass–the nucleus of an atom. Atoms may gain or lose electrons, which change the charge of the atom (creating ions). However, the atom remains the same element whether it has a positive, negative, or neutral charge.
The small, dense nucleus (or center) of the atom contains the other components–the protons and neutrons. Protons are positively charged particles, and the number of protons is always fixed for a particular element. In other words, the number of protons is what gives each element its unique, individual identity. For example, a carbon atom has six protons, but an atom with only five protons is boron while an atom with seven protons is the element nitrogen.
Neutrons are neutral - they have no charge. Isotopes are atoms of the same element that have a different number of neutrons. Although isotopes of the same element are twins when it comes to reactivity, the different number of neutrons means that they have a different mass. Certain isotopes are more abundant in some materials than others since some physical and chemical processes “prefer” one isotope over another. These differences in isotopic abundance are used as “labels” to identify the different sources of CO2 found in an atmospheric CO2 sample. NOAA atmospheric scientists use these isotopic labels to determine what percent of that carbon was derived from fossil fuels, the terrestrial biosphere, or from the ocean.
Isotopes of Carbon
Carbon isotopes come in three forms. By far the most common isotope of carbon is carbon-12 (12C), which contains six neutrons in addition to its six protons. The next heaviest carbon isotope, carbon-13 (13C), has seven neutrons. Both 12C and 13C are called stable isotopes since they do not decay into other forms or elements over time. The rare carbon-14 (14C) isotope contains eight neutrons in its nucleus. Unlike 12C and 13C, this isotope is unstable, or radioactive. Over time, a 14C atom will decay into a stable product.
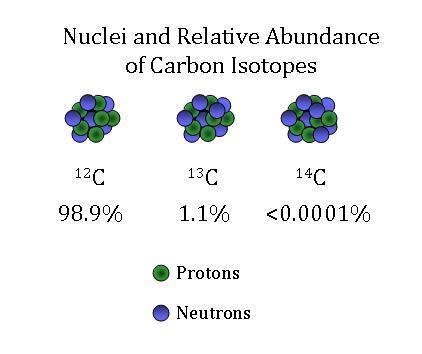
The vast majority of all carbon found on Earth is 12C. Almost 99% of all carbon on Earth is of this form. While only approximately 1% of all carbon on Earth is of the 13C isotopic form, 14C is still much rarer. Only one out of every trillion carbon atoms is 14C.
To gain an idea of how few 14C atoms there are compared to 12C, let's compare one to one trillion. A trillion is a million millions. If you lined up a trillion one dollar bills, it would stretch almost from the Earth to the sun!
